The Energy of an Sp Orbital Will Be:
Each singly occupied sp hybrid orbital can now form an electron-pair bond with the singly occupied 1s atomic orbital of one of the H atoms. Less than that of an s or p orbital greater than that of an s or p orbital less than that of an s orbital but greater than that of a p orbital less than that of an p orbital but greater than that of a s orbital.
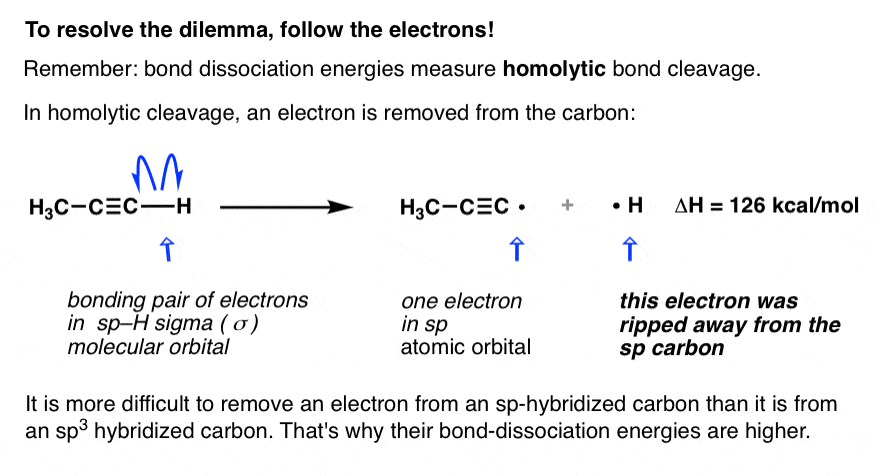
On Hybrid Orbitals And Bond Strengths Master Organic Chemistry
Hence s -s bond is non directional.

. Examples of sp hybrid orbitals are acetylene acetonitrile and allene. In compounds with sp hybrid orbitals many of them will have double or triple bonds. The orbital energy in the same subshell falls as the atomic number Zeff increases.
Each sp hybridized orbital has an equal amount of s and p character 50 s and 50 p character. The first energy level only has room for an S subshell while the second has S. N has one sigma bond to C and the other sp hybrid orbital exists for the lone electron pair.
S P D and F are classified as subshells in atomic theory. If you are in class 11th then dont look for stuff like this. Three atomic orbitals on each carbon the 2s 2p x and 2p y combine to form three sp 2 hybrids leaving the 2p z orbital unhybridized.
Orbitals can be ranked in the increasing order of orbital energy as follows. The formation of two energetically equivalent BeH bonds produces a. Three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals while the remaining electron goes.
1s 2s 2p 3s 3p 3d energy of an electron in multi-electron atoms depends on both on its principal quantum number n and its azimuthal quantum number l. As the n in cespn grows larger we will have more p character and less s character in the hybrid orbital and as explained above it will consequently be higher in energy. As shown in Figure PageIndex3.
We can confirm this intuition by going back. Hydrogen 1s 1 atom has 1s orbital containing a single electron ie. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule.
The energy of an sp orbital will be. From this picture we find an intuitive connection back to where our discussion of orbits started namely the orbital energy. So an cesp hybrid orbital that has 50 s character will be lower in energy than an cesp3 orbital which only contains 25 s character.
According to the Aufbau Principle the energy of an orbital directly impacts the sum of the values of the principal quantum number and azimuthal quantum number. The sigma bonds formed in ethene is by the participation of a different kind of hybrid orbital. Two sp orbitals will be at 180 degrees to each other.
In the molecular orbital energy diagram where should we place the these 2 sp orbitals. I would like to know what is the level of energy of a hybrid orbitalFor instance lets consider the ceN2 molecule. Answer 1 of 7.
1s. The phenomenon of s-p mixing occurs when molecular orbitals of the same symmetry formed from the combination of 2s and 2p atomic orbitals are close enough in. It hasnt been explained for a good reason.
Less than that of a p orbital but greater than that of an s orbital An sp orbital will have an energy in between those of the unhybridized s and porbitals. Each sp orbital on Be has the correct orientation for the major lobes to overlap with the 1s atomic orbital of an H atom. In an sp2 hybridization one s orbital is mixed with two p orbitals to form three sp2 hybridized orbitals.
Sp hybridization is also called diagonal hybridization. Its apoapsis point moves outwards to less negative maximum values of the gravitational potential. Some examples include the mercury atom in the linear.
These electrons occupy subatomic orbitals. The same can be said for acetonitrile and allene. According to its geometry we know that there is an orbital 2p and 2s that are going to form 2 sp orbitals.
This difference in energy of various subshells residing in the same shell is mainly attributed to the mutual. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. An example of sp3 hybridization can be seen in the carbons in ethane.
P orbitals have a higher energy than that of s orbitals. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented. These sp2 hybridized orbitals are oriented with bond angle of 120 degrees in a trigonal.
They are sets of orbitals all within the same shell which is synonymous with energy level. When atomic orbitals hybridize the valence electrons occupy the newly created orbitals. Would they be in the middle of a 2p and a 2s.
P orbital is an atomic orbital having a dumbbell shape. Both C and N have 2 p orbitals each set aside for the triple bond 2 pi bonds on top of the sigma. It is half-filledTwo such 1s orbitals from the two hydrogen atoms having electrons with opposite spins approach each other then the potential energy of the system.
UNDERSTANDABLE VERSION- See boy. It describes the angular momentum of electrons in the p orbital. There are two hands from the carbon atoms of acetylene.
The letter p stands for principal. One p orbital can hold a maximum of 6 electrons. The energy of an SP orbital will be.
I will explain it to you in very crude terms but not its role in MOT as you wont be able to understand and. The order followed here is. Factors that affect Orbital Energy.
From this situation we can infer that it is an sp hybrid orbital. This makes HCN a Linear molecule with a. Answer 1 of 2.
18 hours agoWhen Ars Technica space reporter Eric Berger reported the latest delay on Twitter a thread developed in which people chimed in with suggestions that the environmental process was being slow. In sp hybridization the s orbital overlaps with only one p orbital. Clearly as epsilon increases so does the energy of the moving object.
Each of these hybridized orbitals have 33 s character and 67 p character. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. S orbital is spherical in shape and overlapping takes place to some extent in all directions.
The s orbital electron will be more strongly coupled to the nucleus for a given value of the primary quantum number than the p orbital electron which will be more tightly bound in contrast to a d orbital electron. This type of hybridization involves the mixing of one s orbital and one p orbital of equal energy to give a new hybrid orbital known as an sp hybridized orbital.
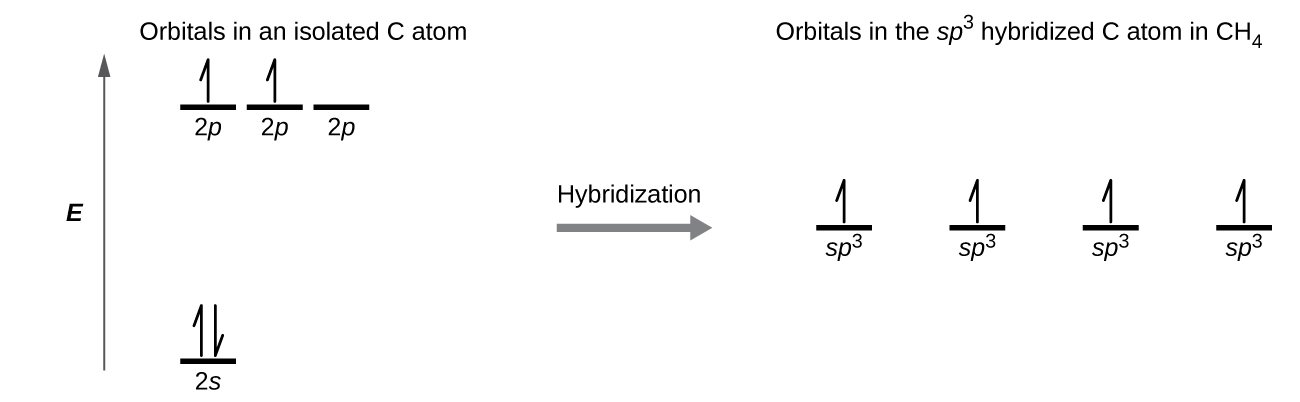
8 2 Hybrid Atomic Orbitals Chemistry

2 The Other Sp Orbitals Hold The Oxygen Lone Pairs

No comments for "The Energy of an Sp Orbital Will Be:"
Post a Comment